Welcome to “A to Z: Drugs in Highlight
This series is designed to delve into the intricate realm of pharmaceutical drugs. As the world of pharmaceuticals continues to evolve with new discoveries and emerging challenges, we aim to shed light on the diverse array of medications available today, ranging from blockbuster medications to those that may not be as familiar. Whether you’re a healthcare professional, a student, or simply someone with a curious mind, “A to Z: Drugs in Highlight” promises to be an enlightening and engaging series.
Y is for YONDELIS®
YONDELIS® (originating from Pharma Mar) is used for treating advanced soft-tissue sarcoma and ovarian cancer.
How it works:
YONDELIS® is indicated for treatment of adult patients with advanced soft-tissue sarcoma. Advanced soft-tissue sarcoma originates from the body’s soft, supportive tissues. The cancer is considered ‘advanced’ when it begins to spread. Efficacy data for YONDELIS® are based mainly on leiomyosarcoma (a cancer that starts in smooth muscle tissue) and liposarcoma (a cancer that starts in the fat cells) patients. YONDELIS® is administered to patients whose cancer has stopped responding to anthracyclines and ifosfamide, or to those who cannot receive these medications.
YONDELIS® is also indicated for treatment of patients with relapsed platinum-sensitive ovarian cancer. YONDELIS® is administered in combination with pegylated liposomal doxorubicin (PLD).
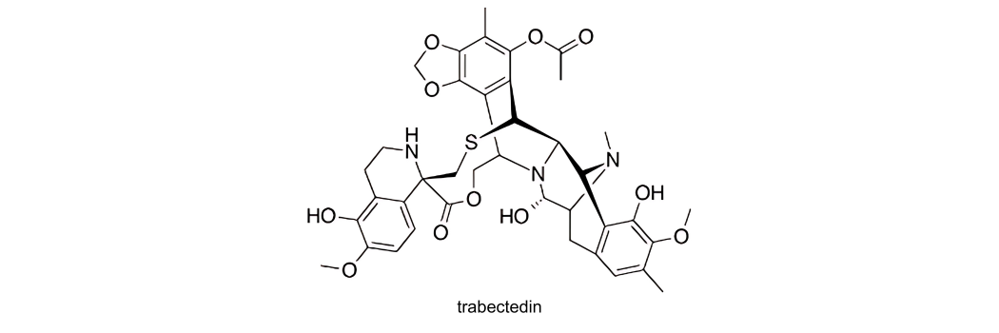
The active substance in YONDELIS® is trabectedin. Cancer occurs when cells divide uncontrollably, often due to genetic malfunctions. Trabectedin combats this by binding to the DNA (the chemical molecule that carries genetic information). By attaching to the DNA, trabectedin interferes with the activity of certain genes, preventing them from becoming overly active. This action helps to slow down the rapid division of cancer cells, thereby inhibiting the growth of the cancer.
Trabectedin is composed of three tetrahydroisoquinoline subunits. It is a synthetic version of substance that was originally extracted from a marine organism known as a tunicate, or ‘sea squirt’
Market impact:
YONDELIS® is approved for marketing in the United States (approved by the FDA in October 2015) and in Europe (approved by the EMA in September 2007). In Europe, YONDELIS® was designated an orphan medicine (a medicine used in rare diseases) in May 2001 (for soft-tissue sarcoma) and in October 2003 (for ovarian cancer).
Patent protection:
There are patent rights protecting various aspects of YONDELIS® in the United States and Europe. For example, in Europe, EP1658848 relates to a composition that comprises an ecteinascidin (a family of biologically active compounds containing two or three tetrahydroisoquinoline subunits) and a disaccharide. Claim 2 of EP1658848 limits the ecteinascidin to ET-743, another name for trabectedin. EP1658848 is due to expire in October 2025.
In the United States, US8895557 relates to a lypholised anti-tumor composition comprising a single active anti-tumor compound and a disaccharide selected from sucrose, lactose and a combination thereof, wherein the anti-tumor compound is ET-743 (trabectedin) and wherein the disaccharide is present in a sufficient amount to inhibit conversion of the ET-743 (trabectedin) into ET-701, such that the ET-743 (trabectedin) composition comprises less than 2% ET-701 after storage of the ET-743 (trabectedin) composition at 5 oC for 3 months.
Patent support from Secerna
Our team has a wealth of experience gained from working with world leaders in the chemistry and pharmaceutical disciplines encompassing new chemical entities, materials science, agricultural chemistry, chemical processes and formulation technology. We have also worked extensively in the medical device materials and carbon nanotechnology sectors.
For intellectual property advice relating to your next project, please get in touch. Our team will be happy to assist. Contact us here.


